Solutions
HEALTH
MOVE. ANALYSE. IMPROVE.
Professional analysis systems for prevention and health promotion
With our versatile analysis systems we help you to make motion analysis in practice easier and more efficient. With our powerful video technology, you can quickly and accurately record all important movement data you need for injury prevention and health promotion. Benefit from our many years of experience in the healthcare sector and let us put together the analysis solution that is right for you.*
CONTEMPLAS offers new possibilities for a differentiated functional diagnostic view.
Dr. Peter Amelung, Head of Functional Diagnostics, Sana Kliniken Sommerfeld
Benefit from...
MARKERLESS
Analyse your patients within minutes by using our fully automated tracking system.
MULTI DEVICE
Combine your video recordings with other measuring devices like force plates, EMG and additional time measuring methods.
HIGH PRECISION
Capture precise data for your objective comprehensive analysis with our various measurement systems and latest video technologies.
Areas of application in health care
Our Systems are used in these fields
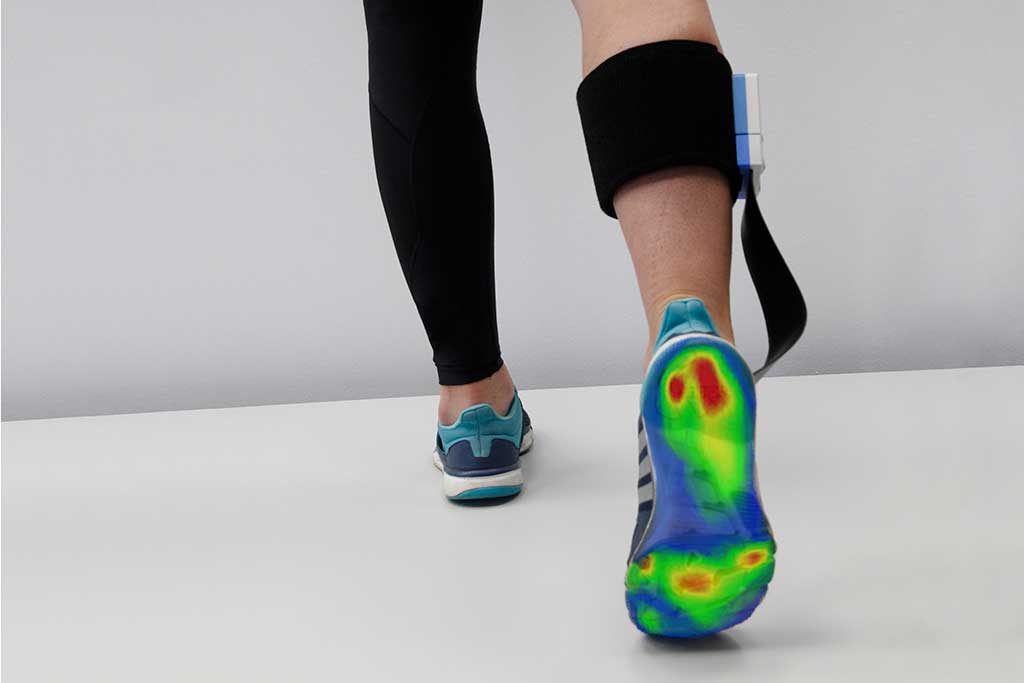
Podometry
Versatile analysis system for functional examination of the musculoskeletal system and gait pattern.
- Analyse changes in the foot, ankle, knee and the static of the leg axes
- Use the measurement data for the fitting of individual shoe insoles
- Check the ACTUAL and TARGET status
Physiotherapy
Analysis, documentation and visualization of functional movements through video-based analysis.
- Detection of asymmetries and movement restrictions
- Identify potential injury risks and inefficient movement patterns
- Optimize training recommendations

Start with your analysis
* No CONTEMPLAS GmbH product is classified as a medical device under MDR (EU) 2017/745 within the European Union, United Kingdom (excluding Northern Ireland) under Part II, UK MDR 2002 (SI 2002 no. 618) or within the requirements of any other national medical regulatory body. CONTEMPLAS GmbH supplied products must not be used where the intention is to provide information which will be used to take decisions with diagnosis or therapeutic purposes.
